By Shelton Becknel
When you get a polymerase chain reaction (PCR) COVID-19 test from Worksite Labs, your job is simple. You book an appointment online, visit our testing site, get your nose swabbed and drive away. Within 24 hours — guaranteed — your results pop up on your phone, and you can hop on a plane, go back to work or school, or do whatever it is you need to do.
But what happens between your test sample being taken and your results being sent?
During this timeframe, your sample goes through a highly sophisticated diagnostic process, overseen by a team of devoted scientists. Let’s take a behind-the-scenes look at how Worksite Labs gets it done.

Our collection team greets patients and walks them through the sample collection process. Samples are sent to the lab staff, and the science begins.
Each PCR test is processed through one of several testing frameworks called assays. Unlike other assays, our preferred assay isn’t bogged down by an hours-long DNA extraction process, yet it still boasts superior accuracy.
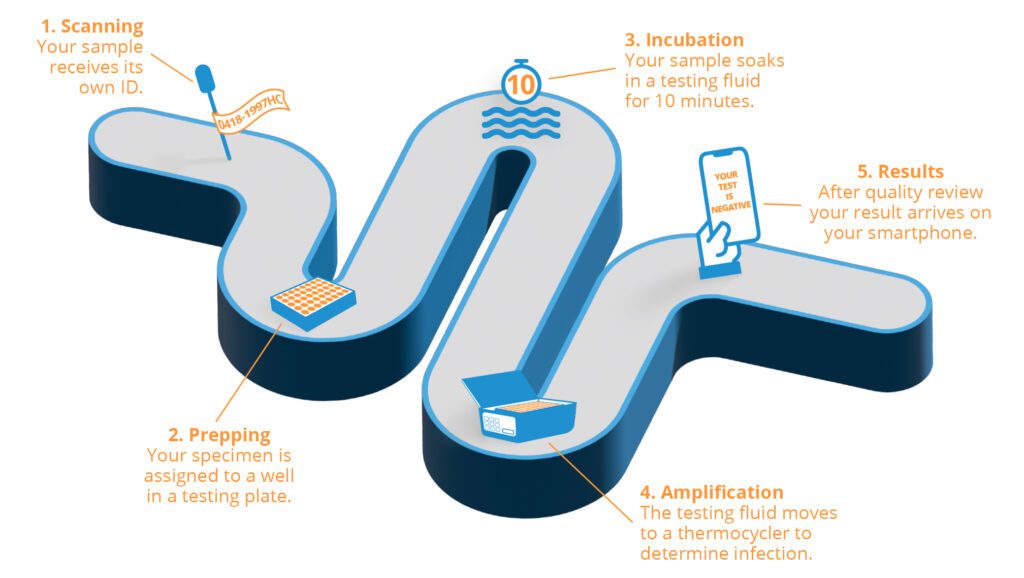
Here’s what happens:
1. Scanning. The specimen is given its own barcode (specimen ID) and scanned into an automated tracking system. Specimen IDs ensure that samples aren’t mixed up and each customer gets his or her own results.
2. Prepping. The sample is then placed in a plate with 93 others; each sample takes up a single cavity, or well, in the plate. In the tracking system, a digital plate map assigns each sample’s specimen ID to the well it’s placed in, so lab staff can track whose test sample goes where.
3. Incubation. All 94 plate wells are filled with a substance called a buffer; the test samples soak in it for 10 minutes. A small amount of the buffer is then extracted from each well and placed into another well in a new plate, called a PCR plate. In the PCR plate, the buffer is combined with a mastermix, a solution that detects signs of coronavirus.
4. Amplification. This is where the science happens. The PCR plate is sealed and placed in a thermocycler. By subtly raising and lowering the temperature of the specimens, this device works to amplify any DNA segments that potentially carry signs of coronavirus infection.
5. Results. Fifteen to 20 minutes later, the tracking system identifies which samples come up positive and which come up negative. After a licensed analyst reviews the results for accuracy, the lab staff sends the 94 test results to our data team to enter into our laboratory management system. Results then are sent digitally to customers.

Of course, that daily work at each lab location only happens after months of work to open each site.
After determining where to open our next Worksite Labs testing site, we decide whether it will be semi-permanent (like many of our airport-adjacent sites) or a pop-up site. Our sites must obtain accreditation through the College of American Pathologists (CAP) as well as federal and state licensure, all of which can take up to six months.
Meanwhile, we’re hiring our collection team and lab staff. Since COVID-19 testing is a high-complexity task, finding qualified lab personnel to perform it is essential.
Each of our lab staffers across the country has a bachelor’s degree in chemistry or biology and undergoes several weeks of training before starting work. We also enable new hires to earn certification through the American Association of Bioanalysts or the American Society of Clinical Pathologists.

Every part of our lab process, from on-site staff practices to testing quality, is closely monitored around the clock, so patients receive top-tier services and the fastest possible turnaround time.
Our labs are enrolled through CAP in an external quality assessment. In this program, CAP sends groups of test samples throughout the year — without telling us which are positive or negative — to monitor our accuracy. Labs must maintain 80% accuracy in reading these test samples; Worksite Labs is currently holding steady at 100% across the board.
“…the level of commitment I saw [in the U.S. Air Force], from coworkers and team members, is the same commitment I see at Worksite Labs.”
We prioritize lab staff’s safety, hygiene and health mitigation practices to keep them safe and prevent compromising test samples. Each lab employee must be fitted for a proper N95 mask every year, as well as wear a gown and nitrile gloves while on the job. And of course, frequent handwashing (including outside of work) is stressed from day one.
Staff are trained to handle all active samples as if they’re infective. They use what are called multichannel pipettes to extract the buffer from multiple specimens at once, reducing the risk of cross-contamination. Meanwhile, workers cannot eat, apply cosmetics or smoke in the labs, as these activities are risky in a potentially infectious environment.
Finally, all on-site employees are trained to respond to natural hazards, accidents and other emergencies. These include procedures for earthquakes, high winds, floods, inclement temperatures, power outages and chemical or sample spills. (Note: It’s always safe to be in business near our testing sites; we uphold proper protocols and keep samples well contained.)

Before joining Worksite Labs, I spent 22 years as a medical technologist in the U.S. Air Force. And the level of commitment I saw during those years, from coworkers and team members, is the same commitment I see at Worksite Labs.
I’m proud to be with this company because everyone here cares so deeply about serving the patient, and because we have the best possible processes in place. Next time you turn to us for a quick test, remember just how much work, focus and care goes into our goal to keep things moving in your life.
Shelton Becknel is vice president of laboratory operations at Worksite Labs.